Blood-Brain Barrier
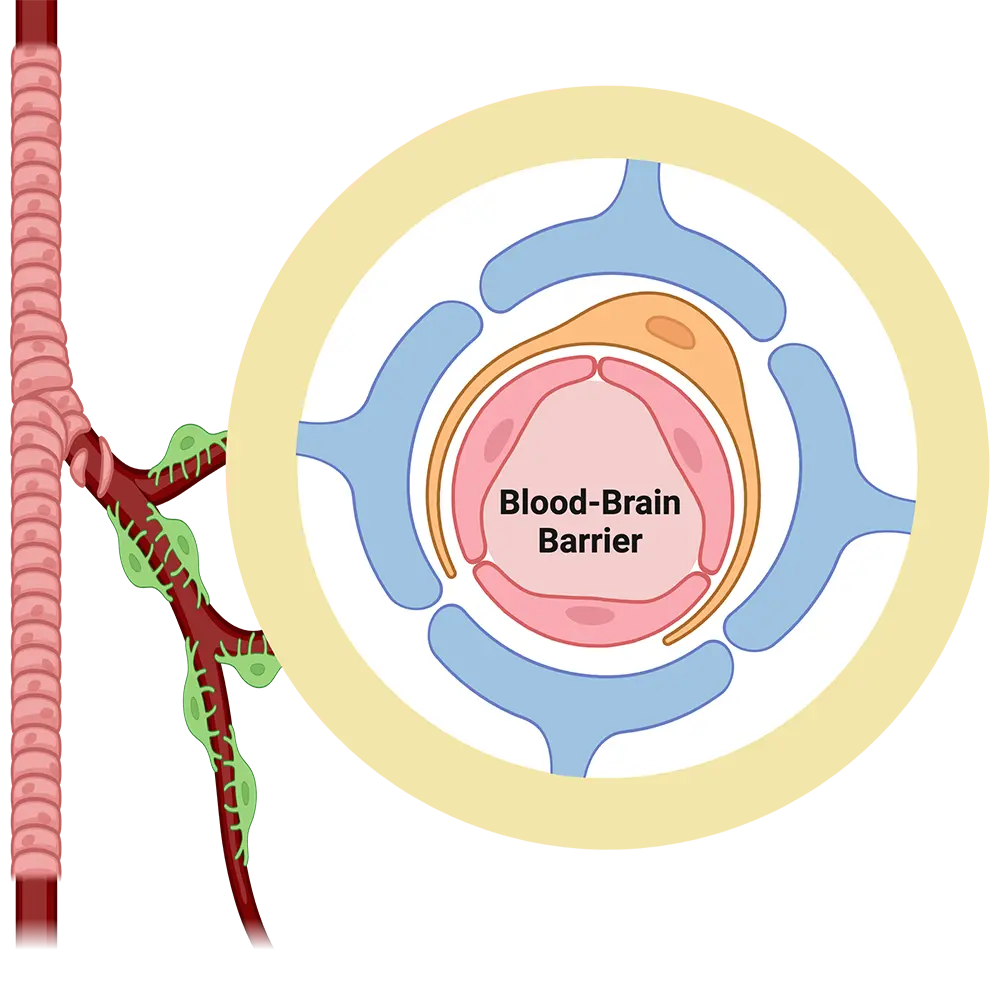
The blood-brain barrier is the interface between blood and brain that controls what goes in and comes out of the brain.
Anatomically, the blood-brain barrier is made of endothelial cells forming a complex vascular network that supplies the brain with oxygen and nutrients and disposes of carbon dioxide and wastes.
The primary role of this barrier is to ensure nutrient supply to the brain and, at the same time, to protect the brain from potentially toxic xenobiotics, including therapeutic drugs. Recent studies show that the blood-brain barrier is affected by brain disorders and itself plays a role in causing brain disease. Therefore, understanding blood-brain barrier function is critical for devising new therapeutic strategies to enhance brain drug delivery, improve brain protection, and treat brain disorders.
Alzheimer’s disease
Millions of people worldwide suffer from Alzheimer’s disease. Patients decline mentally and physically while slowly fading out of life.
Despite intense research efforts, Alzheimer’s disease is still a mystery, and disease-modifying therapies are desperately needed in the clinic.
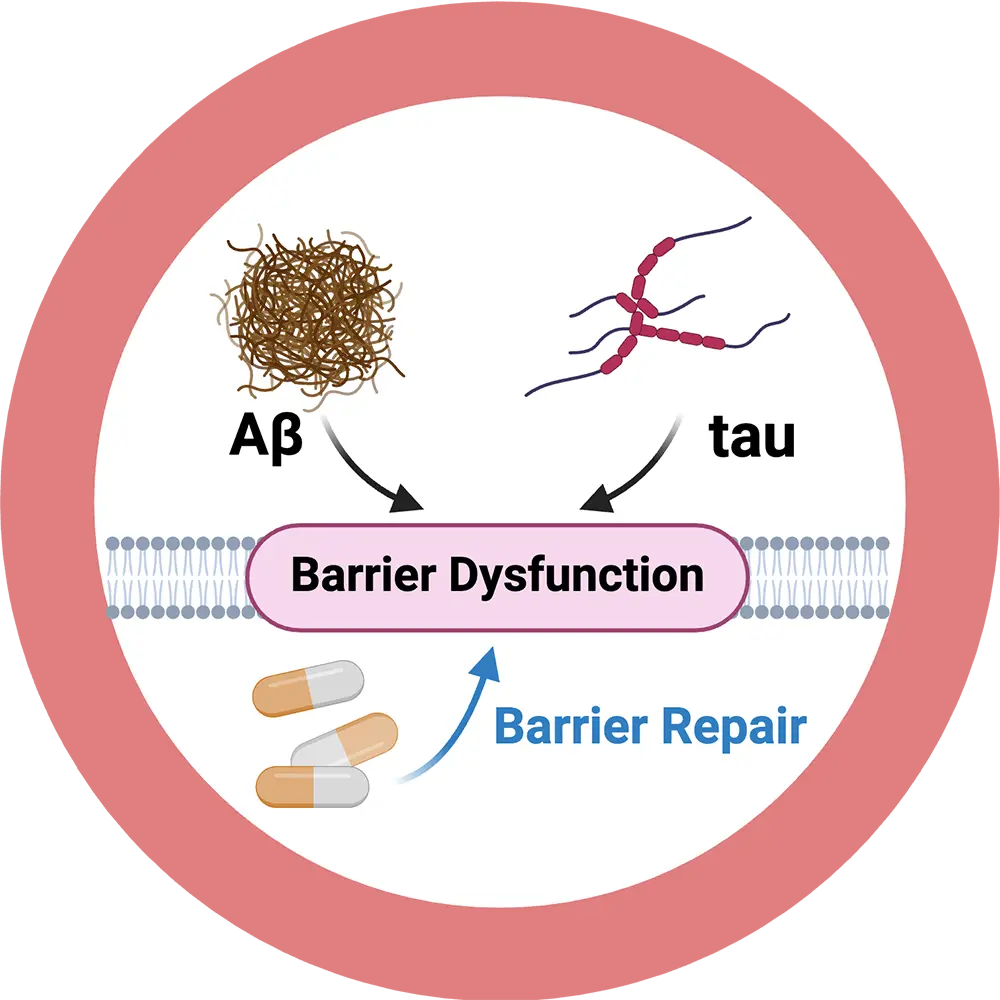
On a molecular level, Alzheimer’s disease is characterized by excessive accumulation of the neurotoxic peptides Aβ and tau in the brain causing neurodegeneration and dementia. We discovered that Aβ and tau are key drivers of blood-brain barrier dysfunction. A leaky and dysfunctional barrier, in turn, contributes to Alzheimer’s disease pathology suggesting a vicious, perpetual feedback loop. However, therapeutic strategies that repair Aβ/tau-induced barrier dysfunction are not available. Our goal is to establish a mechanism-based intervention that targets Aβ/tau to treat barrier dysfunction in Alzheimer’s disease. Our disease-modifying strategy is designed specifically to enhance Aβ and tau removal from the brain, which holds the promise to delay onset and slow progression of Alzheimer’s disease.
Epilepsy
More than 50 million people worldwide suffer from one of the most common neurological diseases: epilepsy.
The main treatment for epilepsy is pharmacotherapy with anti-seizure drugs.
Drug treatment, however, has two major disadvantages:
- Anti-seizure drugs can cause severe side effects that lower quality of life
- About one-third of patients are drug-resistant, do not respond to treatment, and continue to have uncontrolled seizures.
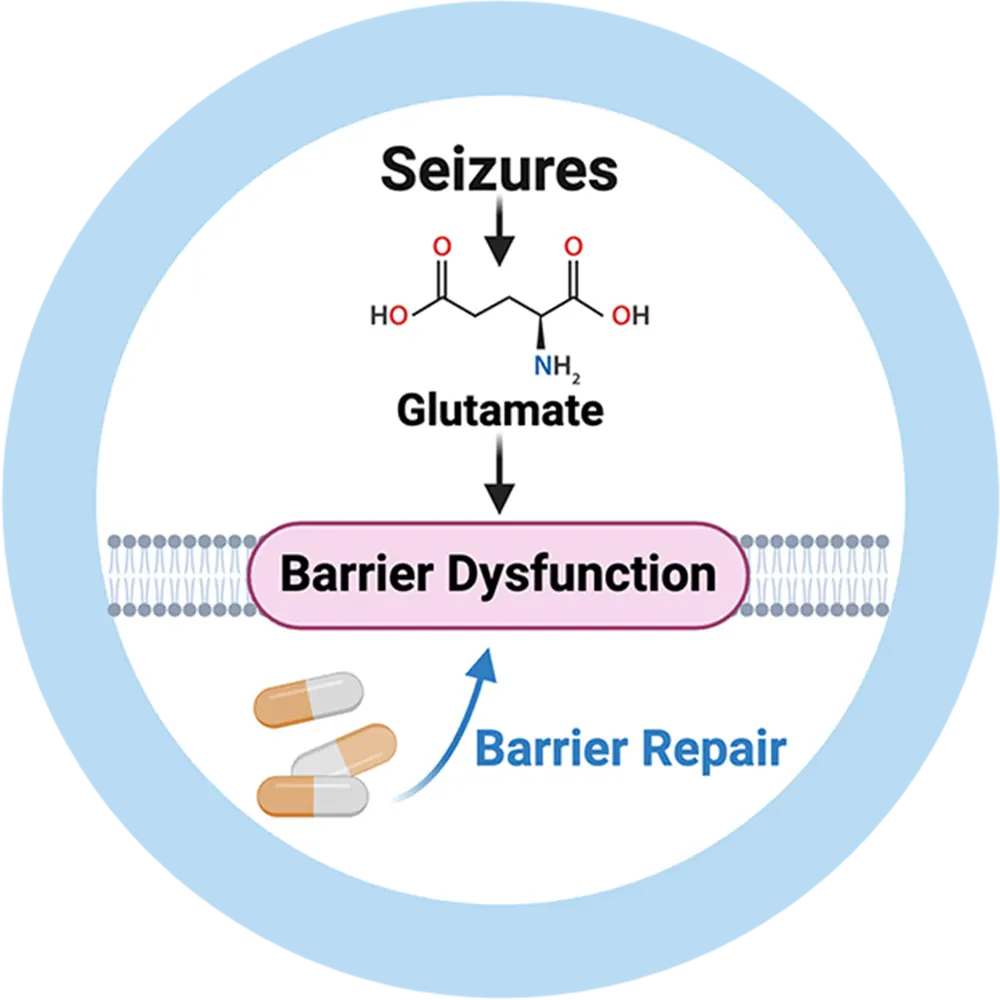
Seizures cause neurovascular inflammation and barrier leakage resulting in barrier dysfunction. Barrier dysfunction, in turn, triggers new seizures, leading to a pernicious cycle that contributes to drug resistance and drives epilepsy pathology.
We are currently evaluating the therapeutic benefit of a novel, patented treatment that targets the underlying mechanism causing barrier dysfunction in epilepsy. Our approach is designed to resolve inflammation and repair barrier dysfunction to overcome drug resistance and reduce seizures to help patients with epilepsy.
Epilepsy Team Science
Seizure burden, especially in patients with poorly controlled, drug-resistant epilepsy, is a serious clinical problem.
In this regard, blood-brain barrier dysfunction is a critical factor that triggers a chain of events leading to seizures and contributes to the development of drug resistance.
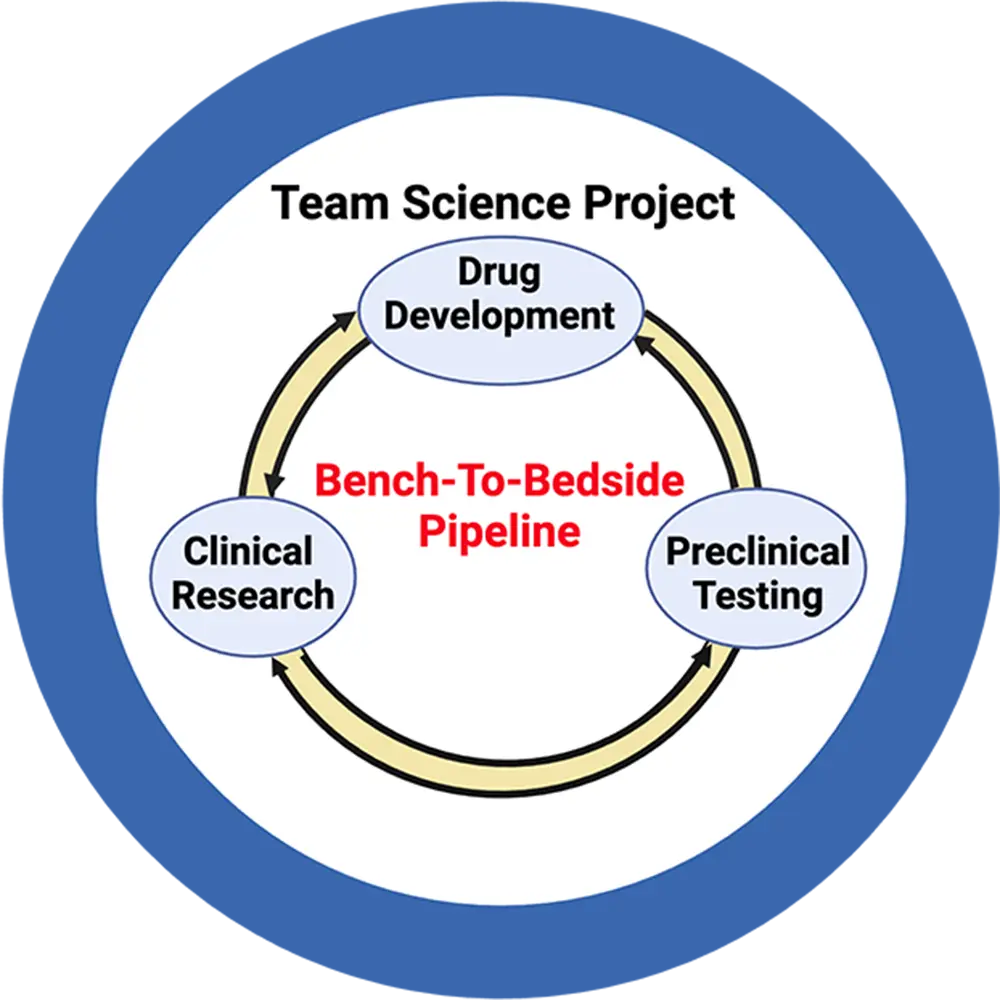
We identified two FDA-approved drugs that attenuate barrier dysfunction in vivo. Our preliminary data suggest that repairing barrier dysfunction helps reduce seizure burden, yet therapeutic options to restore barrier function in patients are currently not available. We directly address this critical unmet need in our team science project by establishing a bench-to-bedside pipeline to develop novel drugs, test these drugs in preclinical epilepsy models, and conduct clinical pilot studies in patients with poorly controlled epilepsy.
Brain Cancer
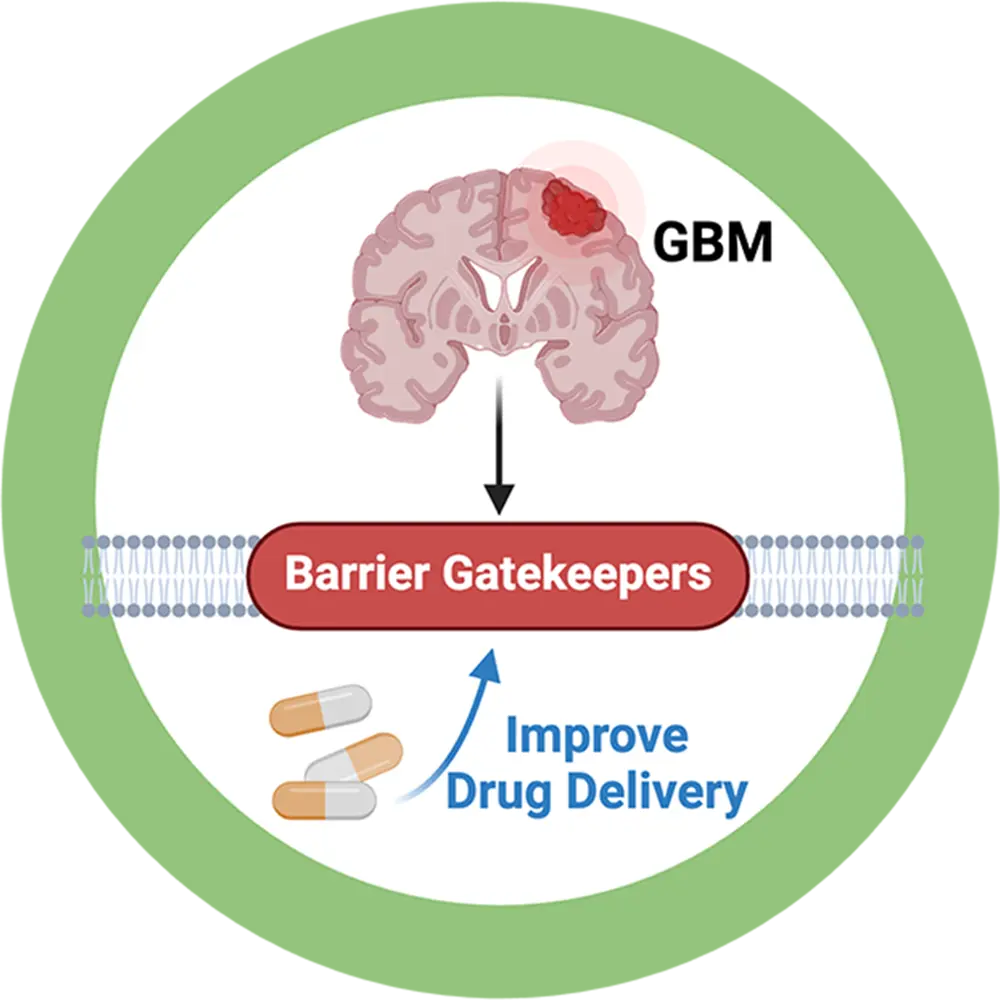
Glioblastoma is a complex, deadly, and treatment-resistant brain tumor.
Despite all efforts, no significant advances have been made in the last decades and the diagnosis of glioblastoma remains a death sentence.
Treatment options for glioblastoma are limited. Surgery and radiation are the primary choices of treatment, but remnant cancer cells that cannot be removed grow into larger and more aggressive tumors. Therefore, post-surgical chemotherapy is critical. However, most chemotherapeutic drugs do not cross the blood-brain and fail to eradicate the tumor cells.
The main problem for successful drug delivery into the brain and tumor tissue is a group of gatekeepers at the blood-brain and blood-tumor barriers. These gatekeepers limit chemotherapeutic drugs from entering the brain and reaching the tumor.
To overcome this clinical obstacle, we focus on targeting a signaling mechanism to turn off these gatekeepers and are currently testing this strategy. Our goal is to increase brain uptake of chemotherapeutic drugs to improve glioblastoma chemotherapy.